Impact of the European regulation on medical devices, seen from Switzerland
Swiss Medtech (industry association for Swiss medical technology) publishes its Medtech sector study 2022. This article summarizes the impact of MDR on Swiss manufacturers
This – very detailed – study includes manufacturers, distributors and suppliers (goods or services); it addresses the economic, strategic, technological and environmental aspects of MD sector.
- Switzerland has become a third country for Europe due to the lack of a regulatory agreement
- This status causes shortages of MD for 82% of importers and distributors
- 20% of manufacturers plan to create jobs in R&D
- 53% of manufacturers plan to create jobs in AR/QA.
- MDR compliance increases R&D costs by 12%, for 79% of manufacturers
- DM prices increase by 6%, on average
- 63% of manufacturers are reducing their product portfolio (13% reduction, on average)
- Increasing quality and documentation requirements is elected “Top challenge for medtech companies”
- Uncertainty with legal interpretations is elected “Top difficulty for manufacturers”
- Increase product prices is voted “Top priority for manufacturers, suppliers and distributors”
- An image summarizes the evolution of access to different markets:
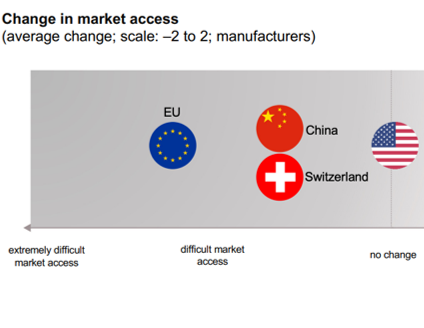
Source : Swiss Medtech
