EN ISO 13485/A11: Annex ZA and ZB for regulations 2017/745 and 2017/746

Amendment A11 to ISO 13485 (Medical devices. Quality management systems. Requirements for regulatory purposes) is available. It includes Annexes ZA and ZB that link the standard to the requirements of Regulations 2017/745 (on medical devices) and 2017/746 (on in vitro diagnostic medical devices).
This annex is a necessary ste^for the harmonization process of 13485.
Context: from ISO 13485 to EN ISO 13485
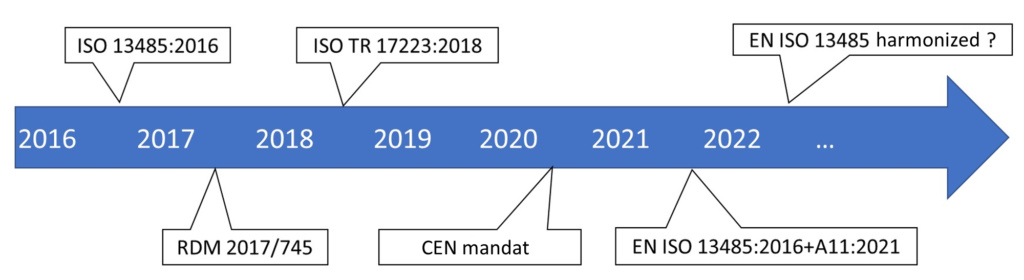
- The ISO 13485 was published in 2016. It enables the implementation of a quality management system (QMS) in a medical device context, for regulatory purposes.
- The Regulation (EU) 2017/745 frames medical devices in Europe. It requires manufacturers to implement a QMS. The MDR (European Medical Device Regulation) was published in May 2017, and is applicable since May 2021.
- The regulation asks that manufacturers to use harmonized standards to achieve conformity.
- A harmonized standard consists in an ISO or IEC standard, complemented by Z-annexes that bridge the gap (convergences and deviations) between the standard and the European regulation.
- The 2018 ISO TR 17223 bridges the gap between ISO 13485:2016 and the requirements of Regulation 2017/745.
- The CEN CENELEC has been mandated since May 2020 to provide harmonized standards for regulations.
- Europe published a first list of harmonized standards to the regulation in July 2021. There are currently 5 out of the 228 planned to save Europe from health dramas.
- The amendment A11 to ISO 13485 incorporates the elements of ISO TR 17223
- Amendment A11 has been available since September 8, 2021, and will be applicable by March 31, 2022.
Contents of A11/Annex ZA: Relationship between the MDR and ISO 13485
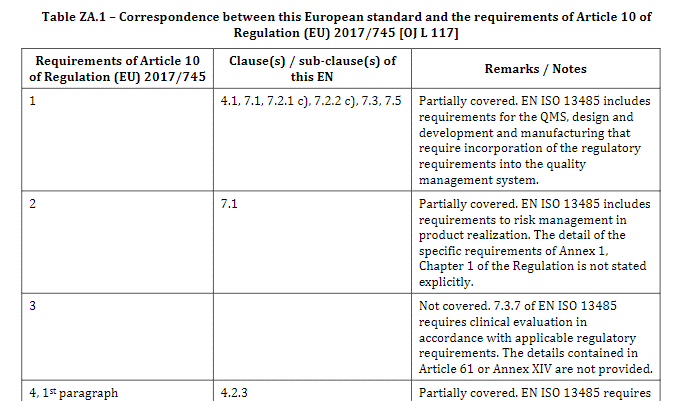
Scope of Annex ZA
Annex ZA compares ISO 13485 to the requirements of Regulation (EU) 2017/745 stated in Article 10 (general requirements on QMS), in Annex IX (evaluation of conformity based on a QMS) and in Annex XI (production quality assurance).
Moreover, the amendment only considers requirements for manufacturers.
Hard points
There are two hard points in annex ZA:
- Risks must be reduced as much as possible, but the idea of the regulation is more to control the risks according to the state of the art, not more.
- The requirement for continuous improvement present in the MDR it is not required by 13485. This point may be a misunderstanding of the intent of the regulation, which speaks of improvements to maintain conformity and a favorable benefit / risk balance.
Where to get Amendment A11?
Amendment A11 is currently available only in English. Other languages have until March 31, 2022 to come forward.
EN ISO 13485:2016+A1:2021 is priced at 328€ by BSI. Fortunately EVS sells EN ISO 13485/A1:2021 (only annex ZA and ZB, not the requirements) for 15€ and rental for 2€.
Source: CEN
