Roles and obligations in the Medical Devices Regulation
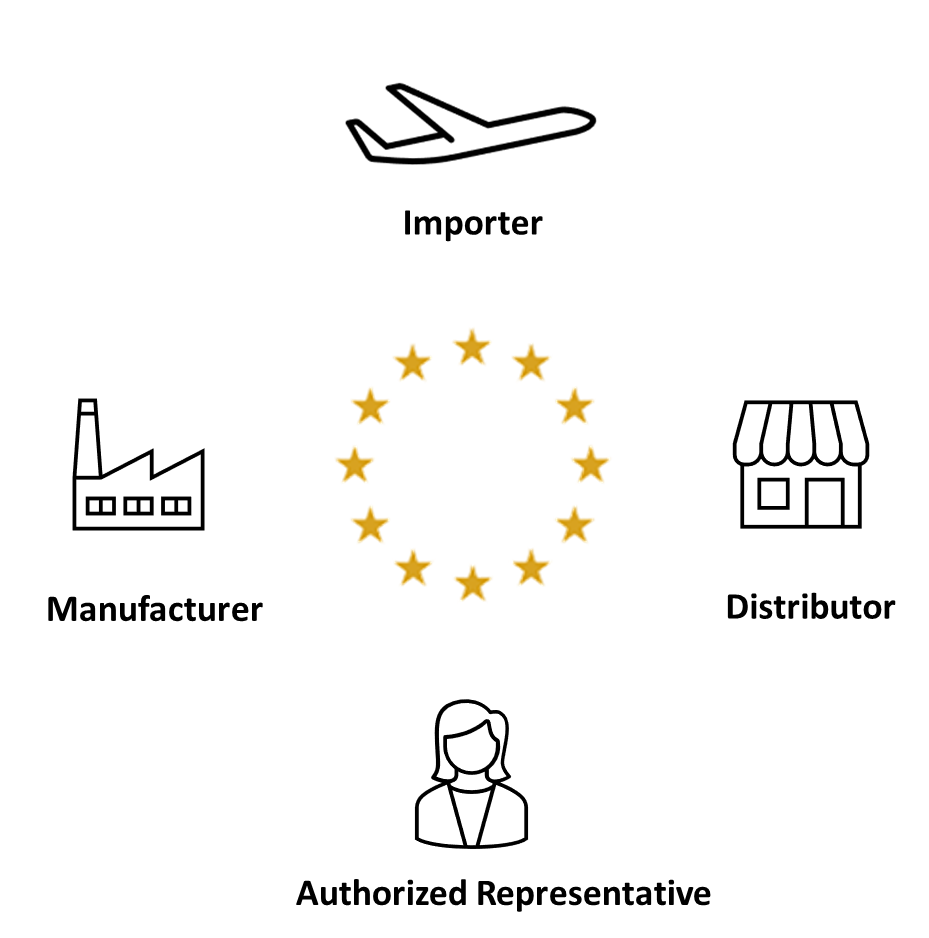
Regulation (EU) 2017/745 defines the obligations of the different economic operators in the life of a medical device.
This article reiterates the definitions and requirements for manufacturers, authorized representatives, importers and distributors.
Four roles are definied in MDR 2017/745
The regulations identify up to 4 roles, not all of which are necessary and a same actor can play different roles.
In the simplest case: a european manufacturer does not need an importer or an authorised representative and can distribute itself the device.
In a more complex case:
- A non-EU manufacturer is responsible for the CE marking of its product.
- An importer places the device on the EU market.
- An authorized representative acts on behalf of the manufacturer for European regulatory tasks.
- A distributor makes the device available to the user.
The regulation emphasizes good cooperation in monitoring devices, a need to “watch each other’s backs” and to feed back post-market surveillance information to the manufacturer and to national authorities.
1. Manufacturer
Definition of medical devices manufacturer
Manufacturer means a natural or legal person who manufactures or fully refurbishes a device or has a device designed, manufactured or fully refurbished, and markets that device under its name or trademark.
Note that the manufacturer does not necessarily manufacture the device: manufacturing and design can be subcontracted (case of OBL / OEM), in other words “it is the one on the label who is responsible”.
Obligations of medical devices manufacturer
The most significant part of the regulation is aimed at the manufacturer, with obligations defined in Chapters II (making MDs available), III (identification and traceability) and V (conformity assessment). The annex are supplemental.
2. Authorized Representative

Definition of authorized representative
Authorised representative means any natural or legal person established within the Union who has received and accepted a written mandate from a manufacturer, located outside the Union, to act on the manufacturer’s behalf in relation to specified tasks with regard to the latter’s obligations under this Regulation.
The authorised representatives are useful for non-EU manufacturers, it is the authorised representative who is contractually obliged to act on its behalf with respect to regulatory obligations (e.g. making marketing declarations).
The appointment of an authorised representative is mandatory, its acceptance in writing is also a requirement.
Obligations of authorized representative
These are defined in Article 11:
- Verifying the CE marking: the manufacturer must have established a EU declaration of conformity, have constituted the technical documentation of the MD, have applied the correct CE marking procedure.
- Keep documentation available to national authorities: the technical file, the declaration of conformity and, if applicable, the certificate of conformity issued by a notified body.
- Get a unique identification number (UDI) for the device.
- Cooperate with competent authority and respond to its requests on behalf of the manufacturer (sample request, risk management, incident reporting).
- Terminate the mandate if the manufacturer fails to meet its obligations and keep the appropriate authorities informed.
3. Importer

Definition of importer
Importer means any natural or legal person established within the Union that places a device from a third country on the Union market.
The importer is most often an authorised representative and/or distributor.
Obligations of importer
According to Article 13:
- Verify the presence of the CE marking and the declaration of conformity, the correct designation of an authorised representative, the conformity of the labelling, the presence of an instruction for use, the allocation and registration of a UDI (Unique Device Identifier) to which he associates his contact details.
- In case of problem of conformity of the device: the importer informs the manufacturer (and its authorised representative) before refusing the import; in case of serious risks or falsification, it must also inform the competent authority.
- Indicates his or her contact information on the MD packaging or documentation.
- Verifies transport and storage conditions.
- Maintains a “record of plains“: non-conforming products, recalls and withdrawals.
- Collaborates with the manufacturer (the authorised representative) and relevant authorities in the event of investigation of complaints.
- Informs the manufacturer in the event of non-conformity, incidents.
4. Distributor

Definition of distributor
Distributor means any natural or legal person in the supply chain, other than the manufacturer or the importer, that makes a device available on the market, up until the point of putting into service.
Medical equipment stores, pharmacies, online sales sites, supermarkets, sports stores, … the panel is very wide, even surprising.
Obligations of distributor
They are defined in Article 14 :
- Verify that the device bears the CE marking, that the CE declaration has been drawn up, that the user documentation is available, that the UDI has been assigned, if applicable: that the importer conforms to the requirements of the regulation.
- Inform the manufacturer/importer/authorized representative in the event of non-conformity.
- Inform the manufacturer/importer/authorized representative/competent authorities in the event of serious risks or falsification.
- Watch for conditions of storage and transportation of devices.
- Perform post-market surveillance: transmit reports to the manufacturer / importer / authorised representative, manage a register of complaints / non-conformities / recalls / withdrawals.
- When applicable, communication to regulatory authorities of requested documents.
